In recent years, more and more e-cigarette manufacturers have moved to Indonesia to open factories, and Indonesia is gradually becoming one of the e-cigarette production bases.
E-cigarettes are legal for sale in Indonesia. Regulatory authorities include the Indonesian Food and Drug Supervisory Agency (BPOM), the Ministry of Trade, the Directorate General of Customs, the Ministry of Industry, and the Ministry of Health. Products must obtain letters of recommendation from the Ministry of Health, the Food and Drug Supervisory Agency (BPOM), and the Ministry of Industry, as well as Indonesian National Standard Certification (SNI). Imports are permitted only by companies with an e-cigarette import permit issued by the API and the Minister of Trade.
In July 2025, the Indonesian Food and Drug Supervisory Agency (BPOM) issued Regulation No. 18 of 2025 on the Regulation of Tobacco Products and E-cigarettes. E-cigarettes are clearly defined as "other processed tobacco products (HPTL)" and regulated alongside tobacco. Whether containing natural or synthetic nicotine, they are subject to this regulation. The regulation was issued on July 26, 2025, with a one-year transition period, and will be mandatory on July 26, 2026.
I. Main Contents of Regulation No. 18 of 2025:
1. Definitions of Tobacco Products and E-cigarettes
Tobacco products refer to products made wholly or partially from tobacco leaves, processed and consumed by burning, heating, vaporizing, smoking, chewing, or any other means of consumption. These include cigarettes, cigars, leaf tobacco, sliced tobacco, solid and liquid tobacco, and other processed tobacco products.
E-cigarettes are defined as "other processed tobacco products (HPTL)" and contain both naturally derived and synthetic nicotine, and are classified as tobacco products.
2. Nicotine Content Testing Requirements
Products of varying flavors, strengths, and formulations must be tested for nicotine content. Nicotine content must be reported to the Administration by a BPOM-accredited laboratory.
Currently, Indonesia has not set a specific nicotine content limit, but there are reports that nicotine limit requirements will be announced before the regulation becomes mandatory in July 2026.
3. Ingredient and Prohibited Additive Testing Requirements
E-cigarette manufacturers are prohibited from using additives unless scientific evidence demonstrates that the additives are harmless to health.
E-liquids must be tested for banned additives before they are marketed and during circulation, with cross-verification by two different laboratories.
Note: The Indonesian Food and Drug Administration is cracking down on e-cigarette products containing ketamine.
4. Packaging Requirements
Packaging must display the following information:
a. Warning image and warning statement
Warning statement: Must include "Contains nicotine" and "Not for sale to persons under 21 or pregnant women."
pernyataan “mengandung nikotin dan tar”
pernyataan “mengandung nikotin”
pernyataan “dilarang menjual atau memberi kepada orang berusia di bawah 21 (dua puluh satu) tahun dan perempuan hamil”
Health warnings must appear on the smallest and largest packaging of each e-cigarette product.
b. Production code, date, month, and year of production
c. Manufacturer's name and address
d. Importer's name and address
e. Tobacco products must be labeled "No safe limit" and "Contains over 7,000 chemicals and over 83 carcinogens."
f. Label the e-liquid ingredients and nicotine content.
g. Each variant must have five warning labels, evenly distributed during production (20% per set).
h. Clearly label the brand name and product type (e-cigarettes may be abbreviated as "REL").
i. Indicate the capacity of the e-cigarette device and the e-liquid content (in ml).
j. Any misleading information, logos, or promotional terms are prohibited.
5. Warning Image and Warning Statement Requirements:
- Print on the top of the package
- Appear on both the front and back
- Occupy 50% of the packaging area
- Begin with "Peringatan" in yellow text on a black background
- Image must be printed in color
- Arial bold font
6. Penalties for Violations
For violations of this regulation, penalties will be tiered according to severity as follows:
Warning → Severe Warning → Product Removal from the Market.
In serious cases, BPOM may recommend further enforcement measures, such as a market ban, to the Ministry of Trade and other relevant authorities.
II. Testing Standards:
E-cigarettes, e-liquids, and heated tobacco products are tested according to the standards issued by the Indonesian Bureau of Standards:
Heated tobacco products: SNI 8946:2021 - Standards for the production of heated tobacco products
E-liquids: SNI 9070:2022 - Standards for the production of heated tobacco products
III. Nicotine Liquid Packaging Requirements:
According to BPOM Regulation No. 28 of 2024:
- For closed systems or disposable cartridges, nicotine liquid is prohibited from being packaged in cartridges with a capacity exceeding 2 ml, and nicotine liquid is prohibited from being packaged in boxes containing more than two cartridges.
- For open or refillable electronic cigarette systems, nicotine liquid is prohibited from being packaged in containers other than 10 and 20 ml.
For solid electronic cigarettes, nicotine liquid is prohibited from being packaged in packs of 20.

IV. Pre-market Application Materials:
All tobacco products must submit a pre-market application to regulatory authorities before they are marketed. Any changes to the product's formula, packaging, manufacturer, etc. require a new application.
The following materials are required for this application:
- Test report from a qualified testing laboratory
- Nicotine content and ingredient list
- Packaging design with five warning pictograms
- Laboratory accreditation certificate
V. Advertising and Channels:
Online: Social media advertising is completely banned, and e-commerce platforms require age verification (21+).
Offline: Sales are prohibited within 200 meters of schools; point-of-sale advertising, promotions, and sponsorships will be strictly restricted.
E-cigarettes are legally sold in Indonesia but require letters of recommendation from the Ministry of Health, the Food and Drug Supervisory Agency (BPOM), and the Ministry of Industry, as well as Indonesian National Standard Certification (SNI). Imports are permitted only by companies with an e-cigarette import permit issued by the API and the Minister of Trade.
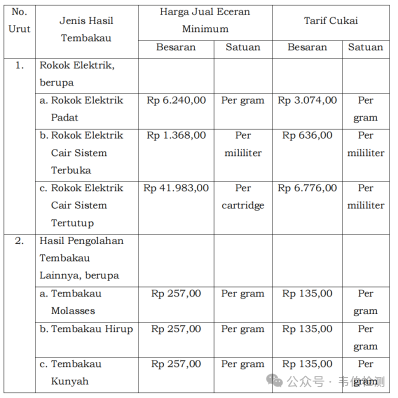
VI. Taxes:
According to Indonesian Ministry of Finance Regulation No. 96 of 2024, starting January 1, 2025, e-cigarettes will be subject to a new minimum retail price plus excise tax, and e-liquids will also be subject to tax labels. In addition to VAT and income tax, e-cigarettes will face significant tax increases:
Heat-not-burn (HNB) cartridges: IDR 6,240 per gram
Open-top e-cigarette liquid: IDR 1,368 per milliliter
Cartridge-type e-cigarettes: IDR 41,983 per cartridge
Additionally, there is a 5% import tariff, 11% VAT, and the corresponding income tax.
VII. What Chinese Manufacturers Should Do:
To ensure legal and compliant e-cigarette products in the Indonesian market, Chinese manufacturers should:
Ensure product specifications are met: Ensure high quality and safety.
Perform product testing: Test in an accredited laboratory according to SNI standards.
Prepare complete technical documentation (ingredients, quality inspection reports, production process, etc.).
Design packaging that meets the requirements of the Indonesian Ministry of Health (reserve a 50% warning area).
Looking for an Indonesian importer partner who meets the following requirements:
A valid API license (importer identification number).
An e-cigarette import approval issued by the Ministry of Trade.
A reliable Indonesian importer with experience in handling the BPOM application process.
If you have any questions, please contact us. We are committed to providing high-quality inspection and certification services.
Contact number:Denny:13128952625
Email address:info@webbtesting.com
WeChat:

Source: Weber Test
Electronic vaporization and HNB products are both new types of electronic devices. Although their structures are small, they integrate a variety of materials, surface treatment, chip electronics, and other technical processes. Moreover, the technology of atomization is constantly evolving, and the supply chain is gradually improving. In order to promote a good docking and communication among enterprises in the supply chain, Aibang has established an industrial WeChat group communication platform, which is open for joining:


电子雾化与HNB产品都是新型电子产品,结构虽小,却融合应用多种材料、表面处理、芯片电子等技术工艺,而且雾化技术一直在不断更迭,供应链在逐步完善,为了促进供应链企业间有一个良好的对接交流,艾邦搭建产业微信群交流平台,欢迎加入;Vape e-cigarettes (VAPE) and Heat-Not-Burn e-cigarettes (HNB) are both emerging electronic products. Despite their compact size, they integrate various materials, surface treatment technologies, chip electronics, and other advanced technical processes. Moreover, atomization technology is constantly evolving and the supply chain is being progressively perfected. To facilitate good communication and networking among supply chain enterprises, Aibang has established an industry WeChat group communication platform and warmly welcomes interested enterprises to join.

